Glycerol With Three Fatty Acids

A triglyceride (TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids (from tri- and glyceride).[1] Triglycerides are the main constituents of body fatty in humans and other vertebrates, as well equally vegetable fat.[2] They are as well present in the blood to enable the bidirectional transference of adipose fatty and blood glucose from the liver, and are a major component of homo pare oils.[3]
Many types of triglycerides exist. One specific nomenclature focuses on saturated and unsaturated types. Saturated fats have no C=C groups; unsaturated fats characteristic one or more C=C groups. Unsaturated fats tend to have a lower melting signal than saturated analogues; as a result, they are often liquid at room temperature.
Chemical structure [edit]
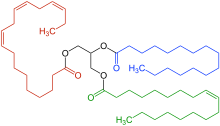
Example of a natural triglyceride with three dissimilar fatty acids. Ane fatty acrid is saturated ( blue highlighted), another contains one double bond within the carbon chain ( dark-green highlighted). The third fat acrid (a polyunsaturated fatty acid, highlighted in ruby-red ) contains 3 double bonds within the carbon chain. All carbon-carbon double bonds shown are cis isomers.
Triglycerides are tri-esters consisting of a glycerol leap to three fatty acrid molecules. Alcohols have a hydroxyl (HO–) group. Organic acids have a carboxyl (–COOH) group. Alcohols and organic acids join to form esters. The glycerol molecule has three hydroxyl (HO–) groups and each fatty acrid has a carboxyl group (–COOH). In triglycerides, the hydroxyl groups of the glycerol join the carboxyl groups of the fatty acrid to class ester bonds:
- HOCH2CH(OH)CH2OH + RCO2H + R′CO2H + R″COtwoH → RCO2CH2CH(OiiCR′)CH2CO2R″ + 3H2O
The three fatty acids (RCO2H, R′CO2H, R″CO2H in the in a higher place equation) are unremarkably different, as many kinds of triglycerides are known. The chain lengths of the fatty acids in naturally occurring triglycerides vary, but most comprise 16, 18, or 20 carbon atoms. Natural fatty acids found in plants and animals are typically equanimous of only even numbers of carbon atoms, reflecting the pathway for their biosynthesis from the two-carbon building-block acetyl CoA. Bacteria, notwithstanding, possess the power to synthesise odd- and branched-chain fatty acids. As a result, ruminant creature fat contains odd-numbered fatty acids, such as xv, due to the action of bacteria in the rumen. Many fat acids are unsaturated; some are polyunsaturated (e.g., those derived from linoleic acrid).[4]
Most natural fats contain a complex mixture of individual triglycerides. Because of this, they cook over a broad range of temperatures. Cocoa butter is unusual in that it is composed of only a few triglycerides, derived from palmitic, oleic, and stearic acids in the ane-, 2-, and 3-positions of glycerol, respectively.[4]
Homo- and heterotriglycerides [edit]
The simplest triglycerides are those where the three fat acids are identical. Their names indicate the fatty acrid: stearin derived from stearic acrid, palmitin derived from palmitic acrid, etc. These compounds can be obtained in three crystalline forms (polymorphs): α, β, and β′, the iii forms differing in their melting points.[four] [5]
If the first and third fatty acids on the glycerol differ, then the triglyceride is chiral.[six]
Conformation [edit]
The shape of fat and fatty acid molecules is ordinarily not well-defined. Whatever two parts of a molecule that are connected by just one single bond are free to rotate almost that bail. Thus a fatty acid molecule with due north simple bonds can be deformed in n-ane independent ways (counting as well rotation of the last methyl group).
Such rotation cannot happen across a double bond, except by breaking and then reforming it with ane of the halves of the molecule rotated by 180 degrees, which requires crossing a pregnant energy barrier. Thus a fat or fatty acid molecule with double bonds (excluding at the very end of the chain) can accept multiple cis–trans isomers with significantly unlike chemical and biological properties. Each double bail reduces the number of conformational degrees of freedom by ane. Each triple bond forces the four nearest carbons to lie in a direct line, removing 2 degrees of freedom.
It follows that depictions of "saturated" fatty acids with no double bonds (like stearic) having a "straight zig-zag" shape, and those with i cis bond (like oleic) being bent in an "elbow" shape are somewhat misleading. While the latter are a little less flexible, both can be twisted to assume similar straight or elbow shapes. In fact, outside of some specific contexts similar crystals or bilayer membranes, both are more probable to be establish in randomly contorted configurations than in either of those two shapes.
Examples [edit]
| Stearic acrid saturated | |
|---|---|
| Oleic acid unsaturated cis-8 |  |
| Elaidic acrid unsaturated trans-8 | |
| Vaccenic acid unsaturated trans-11 | |
Stearic acid is a saturated fat acid (with merely single bonds) institute in animal fats, and is the intended product in full hydrogenation.
Oleic acrid has a double bond (thus being "unsaturated") with cis geometry about midway in the concatenation; it makes up 55–eighty% of olive oil.
Elaidic acrid is its trans isomer; it may exist present in partially hydrogenated vegetable oils, and likewise occurs in the fatty of the durian fruit (virtually 2%) and in milk fat (less than 0.1%).
Vaccenic acid is some other trans acid that differs from elaidic only in the position of the double bail; information technology too occurs in milk fat (about 1–2%).
Nomenclature [edit]
Common fat names [edit]
Fats are usually named after their source (like olive oil, cod liver oil, shea butter, tail fat) or have traditional names of their own (like butter, lard, ghee, and margarine). Some of these names refer to products that contain substantial amounts of other components besides fats proper.
Chemical fatty acid names [edit]
In chemistry and biochemistry, dozens of saturated fatty acids and of hundreds of unsaturated ones have traditional scientific/technical names usually inspired by their source fats (butyric, caprylic, stearic, oleic, palmitic, and nervonic), merely sometimes their discoverer (mead, osbond).
A triglyceride would then be named as an ester of those acids, such every bit "glyceryl i,2-dioleate 3-palmitate".[vii]
IUPAC [edit]
In the general chemical classification developed past the International Union of Pure and Practical Chemistry (IUPAC), the recommended name of a fatty acid, derived from the name of the corresponding hydrocarbon, completely describes its construction, past specifying the number of carbons and the number and position of the double bonds. Thus, for case, oleic acid would be called "(9Z)-octadec-9-enoic acid", pregnant that it has an eighteen carbon concatenation ("octadec") with a carboxyl at 1 end ("oic") and a double bail at carbon 9 counting from the carboxyl ("9-en"), and that the configuration of the unmarried bonds adjacent to that double bail is cis ("(9Z)") The IUPAC nomenclature tin can also handle branched chains and derivatives where hydrogen atoms are replaced by other chemic groups.
A triglyceride would then be named according to general ester rules as, for example, "propane-i,2,3-tryl i,2-bis((9Z)-octadec-9-enoate) 3-(hexadecanoate)".
Fat acrid lawmaking [edit]
A notation specific for fatty acids with unbranched concatenation, that is as precise equally the IUPAC one only easier to parse, is a code of the class "{N}:{D} cis-{CCC} trans-{TTT}", where {Due north} is the number of carbons (including the carboxyl one), {D} is the number of double bonds, {CCC} is a list of the positions of the cis double bonds, and {TTT} is a list of the positions of the trans bonds. Either listing and the label is omitted if there are no bonds of that blazon.
Thus, for example, the codes for stearic, oleic, elaidic, and vaccenic acids would be "18:0", "18:1 cis-nine", "18:1 trans-nine", and "eighteen:1 trans-eleven", respectively. The code for α-oleostearic acrid, which is "(9E,11E,13Z)-octadeca-ix,11,13-trienoic acrid" in the IUPAC nomenclature, has the code "18:three trans-9,xi cis-13"
Nomenclature [edit]
By chain length [edit]
Fats tin be classified according to the lengths of the carbon chains of their elective fat acids. Most chemical properties, such as melting point and acidity, vary gradually with this parameter, so at that place is no abrupt segmentation. Chemically, formic acid (i carbon) and acetic acid (2 carbons) could be viewed equally the shortest fatty acids; and so triformin would be the simplest triglyceride. However, the terms "fatty acid" and "fat" are usually reserved for compounds with essentially longer chains.[ citation needed ]
A partition commonly made in biochemistry and nutrition is:[ commendation needed ]
- Curt-chain fatty acid (SCFA) with fewer than six carbons (e. k. butyric acid).
- Medium-chain fatty acrid (MCFA) with half-dozen to 12 carbons (e.grand. capric acid).
- Long-chain fat acids (LCFA) with 13 to 21 carbons (due east.thou. petroselinic acid).
- Very long concatenation fatty acids (VLCFA) with 22 or more carbons (e. g. cerotic acid with 26)
A triglyceride molecule may have fat acid elements of different lengths, and a fatty product will often be a mix of diverse triglycerides. Well-nigh fats found in nutrient, whether vegetable or animate being, are made up of medium to long-chain fat acids, usually of equal or nearly equal length.
Saturated and unsaturated fats [edit]
For human diet, an important nomenclature of fats is based on the number and position of double bonds in the constituent fatty acids. Saturated fatty has a predominance of saturated fatty acids, without whatever double bonds, while unsaturated fat has predominantly unsaturated acids with double bonds. (The names refer to the fact that each double bail means two fewer hydrogen atoms in the chemical formula. Thus, a saturated fatty acid, having no double bonds, has the maximum number of hydrogen atoms for a given number of carbon atoms – that is, it is "saturated" with hydrogen atoms.)[viii] [ix]
Unsaturated fatty acids are further classified into monounsaturated (MUFAs), with a single double bond, and polyunsaturated (PUFAs), with ii or more.[viii] [nine] Natural fats normally contain several different saturated and unsaturated acids, even on the aforementioned molecule. For example, in virtually vegetable oils, the saturated palmitic (C16:0) and stearic (C18:0) acrid residues are normally fastened to positions one and iii (sn1 and sn3) of the glycerol hub, whereas the middle position (sn2) is unremarkably occupied by an unsaturated 1, such as oleic (C18:i, ω–nine) or linoleic (C18:2, ω–6).[10])
| | Stearic acid (saturated, C18:0) |
| | Palmitoleic acid (mono-unsaturated, C16:i cis-nine, omega-7) |
| | Oleic acid (mono-unsaturated, C18:1 cis-9, omega-9) |
| | α-Linolenic acrid (polyunsaturated, C18:3 cis-9,12,15, omega-three) |
| | γ-Linolenic acrid (polyunsaturated, C18:3 cis-6,ix,12, omega-half dozen) |
While it is the nutritional aspects of polyunsaturated fatty acids that are generally of greatest interest, these materials also have non-food applications. They include the drying oils, such as linseed (flax seed), tung, poppyseed, perilla, and walnut oil, which polymerize on exposure to oxygen to form solid films, and are used to brand paints and varnishes.
Saturated fats generally have a higher melting point than unsaturated ones with the same molecular weight, and thus are more likely to be solid at room temperature. For instance, the animal fats tallow and lard are loftier in saturated fatty acrid content and are solids. Olive and linseed oils on the other hand are unsaturated and liquid. Unsaturated fats are prone to oxidation by air, which causes them to become rancid and inedible.
The double bonds in unsaturated fats can be converted into single bonds by reaction with hydrogen effected by a catalyst. This process, called hydrogenation, is used to turn vegetable oils into solid or semisolid vegetable fats similar margarine, which can substitute for tallow and butter and (unlike unsaturated fats) can be stored indefinitely without condign rancid. Still, partial hydrogenation as well creates some unwanted trans acids from cis acids.[xi]
In cellular metabolism, unsaturated fat molecules yield slightly less energy (i.east., fewer calories) than an equivalent amount of saturated fat. The heats of combustion of saturated, mono-, di-, and tri-unsaturated 18-carbon fatty acid esters have been measured as 2859, 2828, 2794, and 2750 kcal/mol, respectively; or, on a weight footing, x.75, 10.71, x.66, and 10.58 kcal/one thousand – a decrease of about 0.6% for each additional double bail.[12]
The greater the caste of unsaturation in a fatty acid (i.e., the more double bonds in the fatty acid) the more than vulnerable it is to lipid peroxidation (rancidity). Antioxidants tin can protect unsaturated fat from lipid peroxidation.
Cis and trans fats [edit]
Another important classification of unsaturated fatty acids considers the cis–trans isomerism, the spatial arrangement of the C–C unmarried bonds side by side to the double bonds. Most unsaturated fat acids that occur in nature have those bonds in the cis ("aforementioned side") configuration. Partial hydrogenation of cis fats can plow some of their fatty acids into trans ("opposite sides") multifariousness.
Elaidic acid is the trans isomer of oleic acrid, one of the well-nigh mutual fat acids in homo diet. The single change of configuration in ane double bail causes them to have different chemic and physical properties. Elaidic acid has a much higher melting point than oleic acid, 45 °C instead of thirteen.4 °C. This difference is ordinarily attributed to the supposed ability of the trans molecules to pack more tightly, forming a solid that is more than hard to break autonomously.[xiii]
Omega number [edit]
Another nomenclature considers the position of the double bonds relative to the cease of the chain (opposite to the carboxyl group). The position is denoted by "ω−thou" or "n−g", meaning that there is a double bond betwixt carbons k and k+1 counted from 1 at that terminate. For case, alpha-Linolenic acid is a "ω−3" or "due north−three" acid, meaning that at that place is a double bond between the 3rd and fourth carbons, counted from that end; that is, its structural formula ends with –CH=CH–CH
2 –CH
three .[xiv]
Examples of saturated fatty acids [edit]
Some common examples of fatty acids:
- Butyric acrid with iv carbon atoms (contained in butter)
- Lauric acid with 12 carbon atoms (contained in coconut oil, palm kernel oil, and breast milk)
- Myristic acid with 14 carbon atoms (contained in cow's milk and dairy products)
- Palmitic acid with 16 carbon atoms (contained in palm oil and meat)
- Stearic acid with 18 carbon atoms (as well independent in meat and cocoa butter)
Examples of unsaturated fatty acids [edit]
- Myristoleic acid C14:i, ω−5, cis-ix-tetradecenoic acid
- Sapienic acid C16:one ω−10, cis-half dozen-Hexadecenoic acid
- Palmitoleic acrid C16:1, ω−7 , cis-nine-hexadecenoic acrid
- Oleic acrid C18:1 ω−9, cis-9-octadecenoic acid
- Petroselinic acid C18:1 ω−12, cis-Octadec-6-enoic acid
- cis-Vaccenic acid, C18:ane ω−7), cis-11-octadecenoic acrid
- Vaccenic acid C18:1 ω−7, trans-eleven-octadecenoic acid
- Elaidic acrid xviii:ane ω−ix, trans-9-octadecenoic acid (trans-oleic acid)
- Linoleic acid
- Linolenic acid
- Paullinic acid C20:ane ω−7, cis-xiii-eicosenoic acid
- Gadoleic acid C20:i ω−11, cis-9-icosenoic acid
- Gondoic acid twenty:1 ω−9, cis-11-eicosenoic acid
- Erucic acid C22:one ω−9, cis-15-docosenoic acid
- Brassidic acid C22:i ω−9, trans-fifteen-docosenoic acid
- Nervonic acid C24:1 ω−9, | cis-xv-tetracosenoic acid
- Arachidonic acid
Industrial uses [edit]
Linseed oil and related oils are important components of useful products used in oil paints and related coatings. Linseed oil is rich in di- and tri-unsaturated fatty acid components, which tend to harden in the presence of oxygen. This heat-producing hardening procedure is peculiar to these then-called drying oils. Information technology is caused by a polymerization process that begins with oxygen molecules attacking the carbon backbone.
Triglycerides are also split into their components via transesterification during the manufacture of biodiesel. The resulting fatty acid esters can exist used as fuel in diesel engines. The glycerin has many uses, such every bit in the manufacture of food and in the production of pharmaceuticals.
Staining [edit]
Staining for fat acids, triglycerides, lipoproteins, and other lipids is done through the use of lysochromes (fat-soluble dyes). These dyes can allow the qualification of a certain fat of interest past staining the material a specific color. Some examples: Sudan IV, Oil Red O, and Sudan Black B.
Interactive pathway map [edit]
Click on genes, proteins and metabolites below to link to respective manufactures. [§ i]
[[File:

[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]

|alt=Statin Pathway edit]]
- ^ The interactive pathway map can be edited at WikiPathways: "Statin_Pathway_WP430".
Come across also [edit]
- Diglyceride acyltransferase, enzyme responsible for triglyceride biosynthesis
- Medium-chain triglycerides
- Lipid contour
- Lipids
- Vertical auto profile
References [edit]
- ^ "Nomenclature of Lipids". IUPAC-IUB Commission on Biochemical Classification (CBN). Retrieved 2007-03-08 .
- ^ Nelson, D. 50.; Cox, M. M. (2000). Lehninger, Principles of Biochemistry (third ed.). New York: Worth Publishing. ISBN1-57259-153-half-dozen.
- ^ Lampe, M. A.; Burlingame, A. Fifty.; Whitney, J.; Williams, M. 50.; Brownish, B. Eastward.; Roitman, E.; Elias, M. (1983). "Human stratum corneum lipids: characterization and regional variations". J. Lipid Res. 24 (2): 120–130. doi:10.1016/S0022-2275(20)38005-half-dozen. PMID 6833889.
- ^ a b c Alfred Thomas (2002). "Fats and Fatty Oils". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:x.1002/14356007.a10_173. ISBN3527306730.
- ^ Charbonnet, Chiliad. H.; Singleton, W. Southward. (1947). "Thermal backdrop of fats and oils". J. Am. Oil Chem. Soc. 24 (5): 140. doi:x.1007/BF02643296. S2CID 101805872.
- ^ Lok, C.One thousand.; Ward, J.P.; van Dorp, D.A. (1976). "The synthesis of Chiral Glycerides starting from D- and L-serine". Chemistry and Physics of Lipids. sixteen (2): 115–122. doi:10.1016/0009-3084(76)90003-7. PMID 1269065.
- ^ N. Koeniger and H. J. Veith (1983): "Glyceryl-i,2-dioleate-three-palmitate, a breed pheromone of the dear bee (Apis mellifera 50.)". Experientia, volume 39, pages 1051–1052 doi:10.1007/BF01989801
- ^ a b "Essential Fatty Acids". Micronutrient Information Centre, Oregon Land University, Corvallis, OR. May 2014. Retrieved 24 May 2017.
- ^ a b "Omega-three fat acids, fish oil, alpha-linolenic acrid". Mayo Clinic. 2017. Retrieved 24 May 2017.
- ^ Institute of Shortenings and Edible oils (2006). "Food Fats and oils" (PDF). Archived from the original (PDF) on 2007-03-26. Retrieved 2009-02-19 .
- ^ Marchand, V (2010). "Trans fats: What physicians should know". Canadian Paediatric Society. 6 (15): 373–375. doi:10.1093/pch/fifteen.6.373. PMC2921725. PMID 21731420.
- ^ Krisnangkura, Kanit (1991). "Estimation of heat of combustion of triglycerides and fatty acid methyl esters". Journal of the American Oil Chemists' Society. 68: 56–58. doi:10.1007/BF02660311. S2CID 84433984.
- ^ "Section vii: Biochemistry" (PDF). Handbook of chemistry and physics. 2007–2008 (88th ed.). Taylor and Francis. 2007. Retrieved nineteen Nov 2007.
- ^ Karen Dooley (2008): "Omega-three fatty acids and diabetes". Online article at the Academy of Florida'southward UFHealt website. Accessed on 2020-08-30.
External links [edit]
- Lowering Triglycerides (EMedicineHealth.com; October 2020)
Glycerol With Three Fatty Acids,
Source: https://en.wikipedia.org/wiki/Triglyceride
Posted by: simonsdecten.blogspot.com


0 Response to "Glycerol With Three Fatty Acids"
Post a Comment